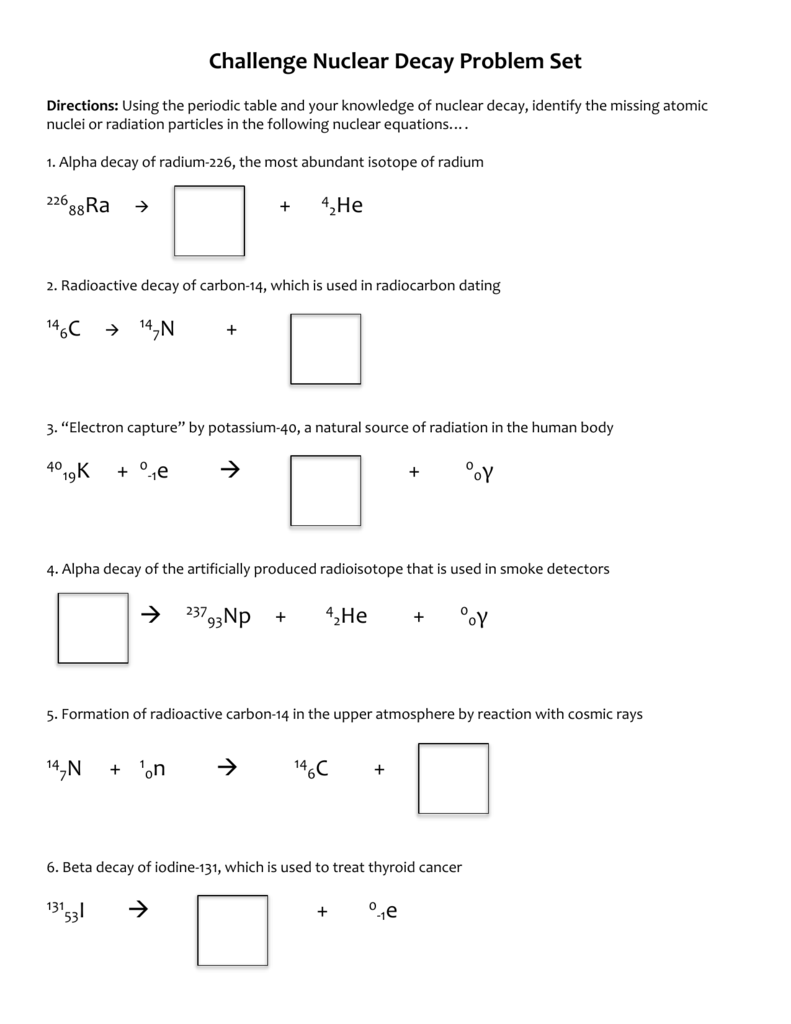
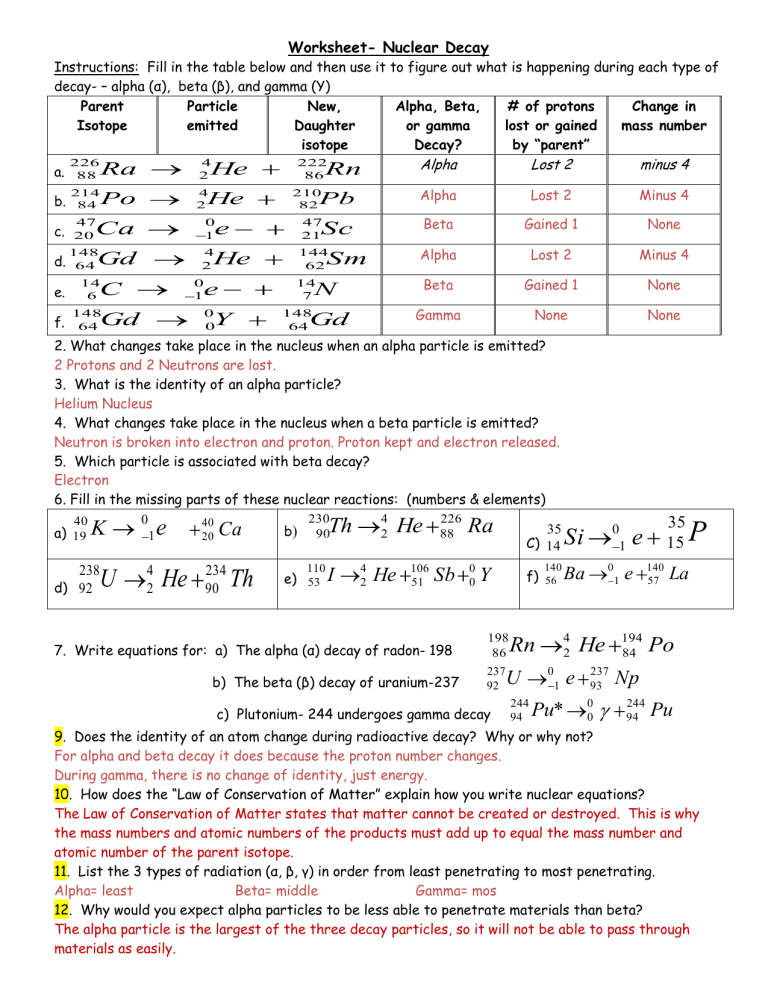
Nuclear Decay Worksheet Answer Key - What is the product of these two decay. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability. Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form.
Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability. What is the product of these two decay.
Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability. Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. What is the product of these two decay.
the student's explanation for nuclear decay and answer key is shown in
Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability. What is the product of these two decay.
Jacob Montoya NuclearDecaySE
Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. What is the product of these two decay. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability.
Nuclear Decay Worksheet Answer Key Worksheet for Education
What is the product of these two decay. Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability.
Radioactive Decay Answer Sheet
Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability. What is the product of these two decay. Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form.
Solved D Worksheet 5 Nuclear Decay Chem & 163 Name Section A
What is the product of these two decay. Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability.
Nuclear Decay Worksheet Chemistry kidsworksheetfun
What is the product of these two decay. Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability.
Nuclear Decay Worksheet With Answers Printable Worksheets and
Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability. What is the product of these two decay.
Worksheet Nuclear Decay
What is the product of these two decay. Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability.
Free Printable Nuclear Decay Worksheets Worksheets Library
Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. What is the product of these two decay. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability.
Nuclear Decay Worksheet Answer Key Ame.my.id
Half‐life is the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. What is the product of these two decay. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability.
Half‐Life Is The Amount Of Time It Takes For Approximately Half Of The Radioactive Atoms In A Sample To Decay Into A More Stable Form.
What is the product of these two decay. Sketch each of the following nuclides on figure 1, calculate their n / z ratios and hence predict their stability.