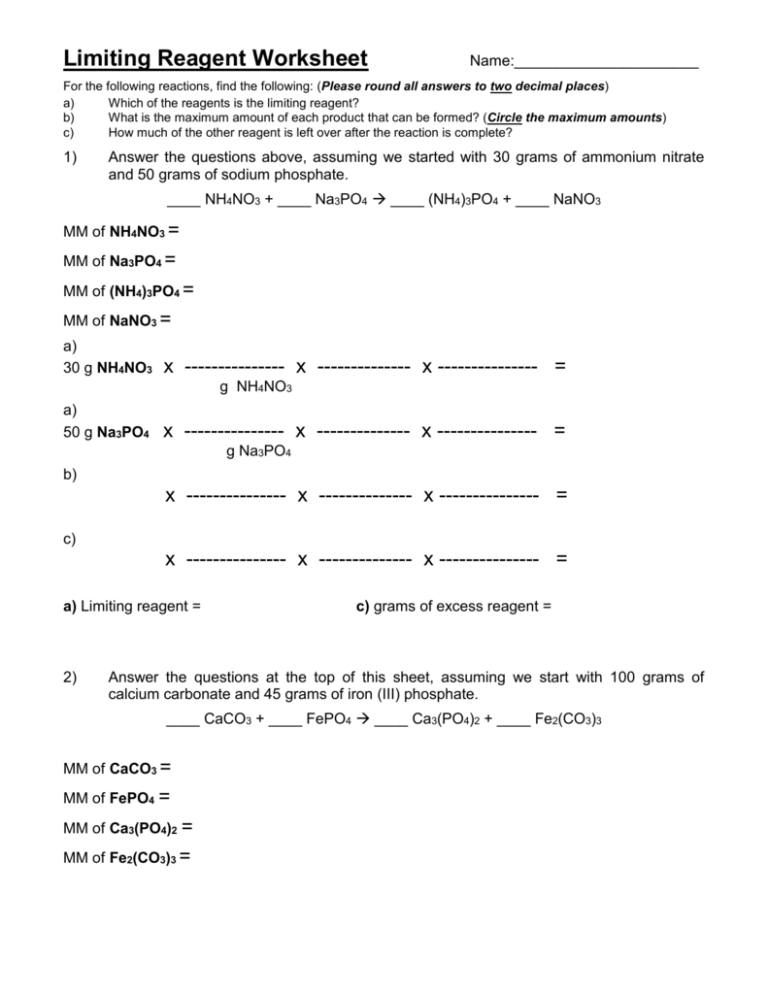
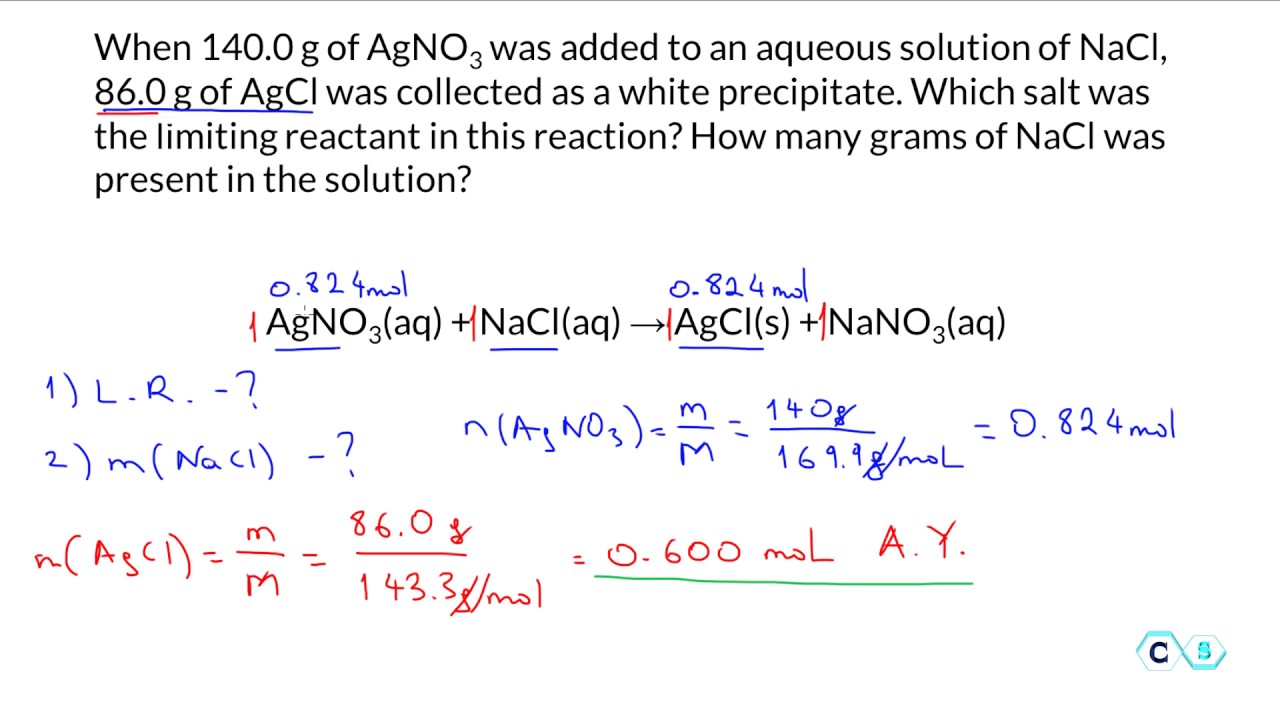Limiting Reagent Worksheet - Because sodium iodide is the reagent that causes 8.51 grams of sodium. If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. Practice identifying the limiting reactant and calculating the product yield in eight chemical reactions. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Determine the number of moles of al produced. What is the limiting reagent in the reaction described in problem 2? Limiting reagent worksheet using your knowledge of stoichiometry and limiting reagents, answer the following questions: Each problem has a balanced.
Each problem has a balanced. If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. Limiting reagent worksheet using your knowledge of stoichiometry and limiting reagents, answer the following questions: What is the limiting reagent in the reaction described in problem 2? Because sodium iodide is the reagent that causes 8.51 grams of sodium. Determine the number of moles of al produced. Practice identifying the limiting reactant and calculating the product yield in eight chemical reactions. How many moles of nh3 can be produced from the reaction of 28 g of n2 ?
If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. Because sodium iodide is the reagent that causes 8.51 grams of sodium. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reagent worksheet using your knowledge of stoichiometry and limiting reagents, answer the following questions: Practice identifying the limiting reactant and calculating the product yield in eight chemical reactions. Determine the number of moles of al produced. What is the limiting reagent in the reaction described in problem 2? Each problem has a balanced.
limiting reagent worksheet answers.notebook
Because sodium iodide is the reagent that causes 8.51 grams of sodium. Limiting reagent worksheet using your knowledge of stoichiometry and limiting reagents, answer the following questions: Each problem has a balanced. What is the limiting reagent in the reaction described in problem 2? If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent.
Limiting Reagent Worksheet 2 Answers
Practice identifying the limiting reactant and calculating the product yield in eight chemical reactions. What is the limiting reagent in the reaction described in problem 2? How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Because sodium iodide is the reagent that causes 8.51 grams of sodium. Determine the number of moles.
Free PrintableLimiting Reactant Worksheets for Students
Because sodium iodide is the reagent that causes 8.51 grams of sodium. Practice identifying the limiting reactant and calculating the product yield in eight chemical reactions. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Determine the number of moles of al produced. If 25.4 g of al2o3 is reacted with 10.2.
Stoichiometry Limiting Reagent Worksheets
Because sodium iodide is the reagent that causes 8.51 grams of sodium. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reagent worksheet using your knowledge of stoichiometry and limiting reagents, answer the following questions: What is the limiting reagent in the reaction described in problem 2? Practice identifying the limiting.
Limiting Reagent Worksheet 1
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Each problem has a balanced. If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. Because sodium iodide is the reagent that causes 8.51 grams of sodium. Determine the number of moles of al produced.
Limiting Reactant Practice Problems Worksheets
Limiting reagent worksheet using your knowledge of stoichiometry and limiting reagents, answer the following questions: Each problem has a balanced. What is the limiting reagent in the reaction described in problem 2? Practice identifying the limiting reactant and calculating the product yield in eight chemical reactions. If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the.
Free Printable Limiting Reagent Worksheets Worksheets Library
Determine the number of moles of al produced. If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. Limiting reagent worksheet using your knowledge of stoichiometry and limiting reagents, answer the following questions: Practice identifying the limiting reactant and calculating the product yield in eight chemical reactions. How many moles of nh3 can be.
Limiting Reactant Example Worksheet
If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Because sodium iodide is the reagent that causes 8.51 grams of sodium. Limiting reagent worksheet using your knowledge of stoichiometry and limiting reagents, answer the following questions:.
Limiting Reagent Worksheet With Solutions
Determine the number of moles of al produced. If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. What is the limiting reagent in the reaction described in problem 2? Each problem has a balanced. Limiting reagent worksheet using your knowledge of stoichiometry and limiting reagents, answer the following questions:
Limiting Reagent Practice Problems With Answers Limiting Rea
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Each problem has a balanced. Practice identifying the limiting reactant and calculating the product yield in eight chemical reactions. If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. Determine the number of moles of al produced.
Each Problem Has A Balanced.
Practice identifying the limiting reactant and calculating the product yield in eight chemical reactions. Because sodium iodide is the reagent that causes 8.51 grams of sodium. Determine the number of moles of al produced. If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent.
What Is The Limiting Reagent In The Reaction Described In Problem 2?
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reagent worksheet using your knowledge of stoichiometry and limiting reagents, answer the following questions: