Iec 62304 Software Development Plan Template - These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. This is an overview over our free templates which. Sopproblemresolution • software development incl. Deliverables, traceability, regular update of. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. The iec 62304 describes how to develop and document software for medical devices. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as.
Sopproblemresolution • software development incl. Deliverables, traceability, regular update of. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. The iec 62304 describes how to develop and document software for medical devices. This is an overview over our free templates which.
Deliverables, traceability, regular update of. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. Sopproblemresolution • software development incl. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. This is an overview over our free templates which. The iec 62304 describes how to develop and document software for medical devices. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution.
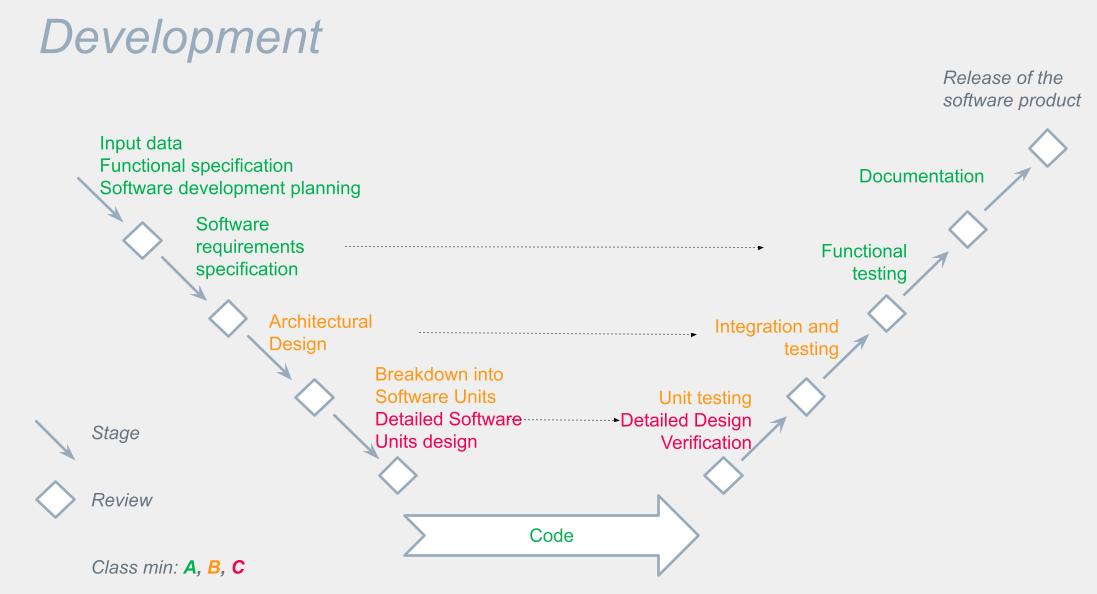
IEC 62304 Medical device Software life cycle
Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. The iec 62304 describes how to develop and document software for medical devices. Iec 62304 software safety classification the.
Iec 62304 Software Development Plan Template
The iec 62304 describes how to develop and document software for medical devices. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. Deliverables, traceability, regular update of. This is an.
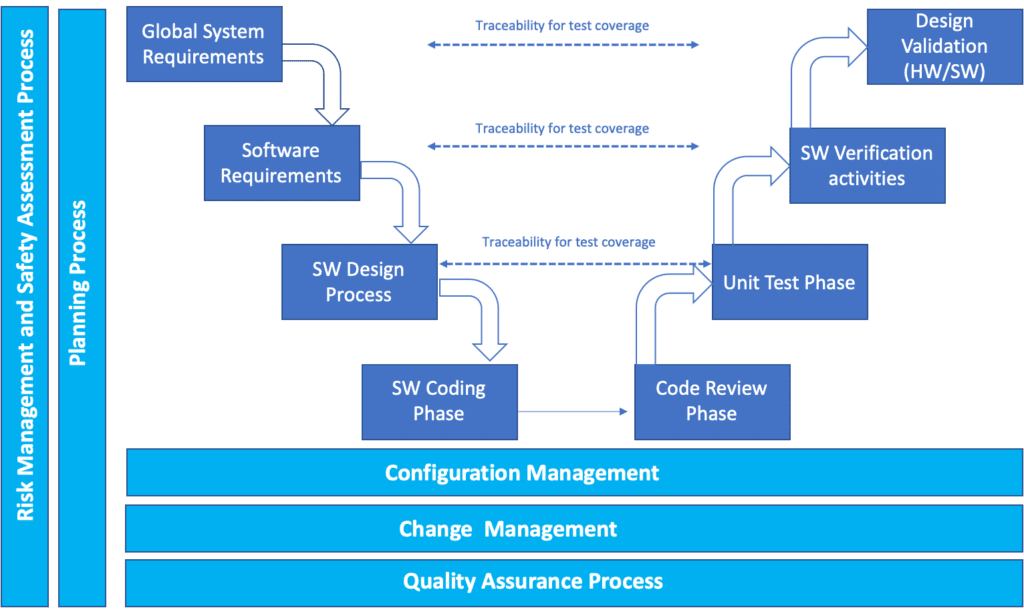
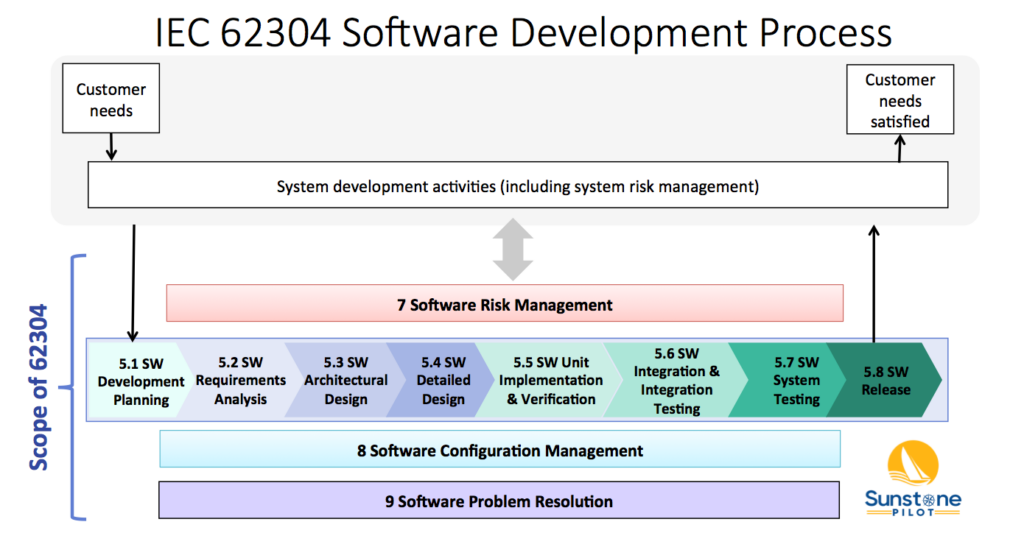
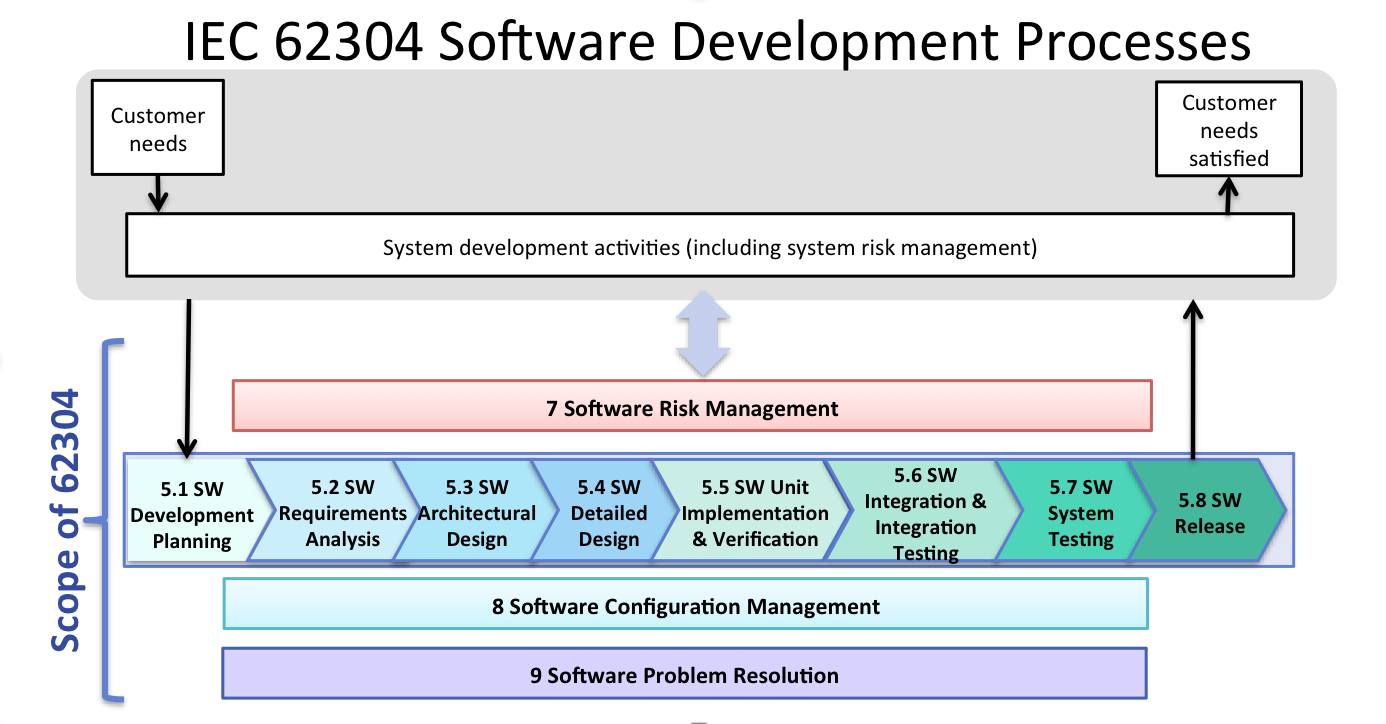
IEC 62304 Software Development Process Sunstone Pilot, Inc.
Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. Sopproblemresolution • software development incl. This is an overview over our free templates which. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. Intland’s medical iec 62304 & iso 14971 template is.
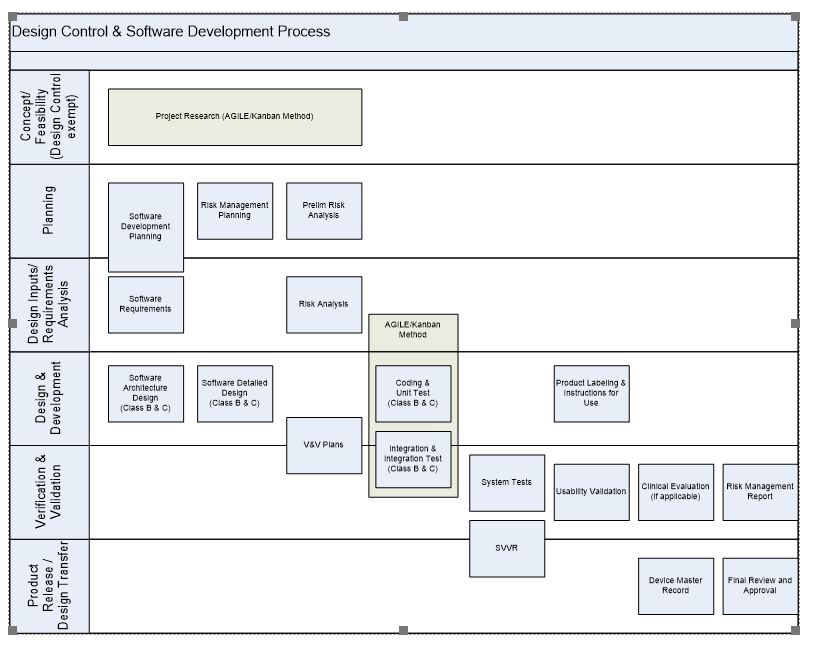
[PDF] Creation of an IEC 62304 compliant software development plan
The iec 62304 describes how to develop and document software for medical devices. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. Sopproblemresolution • software development incl. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. This is an overview over.
IEC62304 Template Software Development Plan PDF
Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments. Sopproblemresolution • software development incl. Iec 62304 software safety classification the software safety classification for [enter device.
Iec 62304 Software Development Plan Template
The iec 62304 describes how to develop and document software for medical devices. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. These forms can be just used as a checklist with notes taken.
Iec 62304 Software Development Plan Template
Deliverables, traceability, regular update of. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to.
FDA Software Guidances and the IEC 62304 Software Standard Sunstone
The iec 62304 describes how to develop and document software for medical devices. This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. These templates deal with sections of iec 62304 about project organisation, software.
Software Development Plan Template MedicalDeviceHQ
This is an overview over our free templates which. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution. The iec 62304 describes how to develop and document software for medical devices. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. Deliverables, traceability, regular update of.
IEC62304 Template Software Development Plan V1 0 PDF Software
This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. Iec 62304 software safety classification the software safety classification for [enter device name] has been established as. Intland’s medical iec 62304 & iso 14971 template is a preconfigured kit that leverages the advanced capabilities of codebeamer alm to help you. These templates.
Intland’s Medical Iec 62304 & Iso 14971 Template Is A Preconfigured Kit That Leverages The Advanced Capabilities Of Codebeamer Alm To Help You.
Deliverables, traceability, regular update of. This is an overview over our free templates which. Sopproblemresolution • software development incl. These templates deal with sections of iec 62304 about project organisation, software configuration and problem resolution.
Iec 62304 Software Safety Classification The Software Safety Classification For [Enter Device Name] Has Been Established As.
This template outlines the overall plan for software development, including the project scope, objectives, resources, schedules, and milestones. The iec 62304 describes how to develop and document software for medical devices. These forms can be just used as a checklist with notes taken separately for document and procedure references and comments.
![[PDF] Creation of an IEC 62304 compliant software development plan](https://d3i71xaburhd42.cloudfront.net/52b435e220f8b1206dfa9d6fa34c19cef7a3135e/5-Table1-1.png)