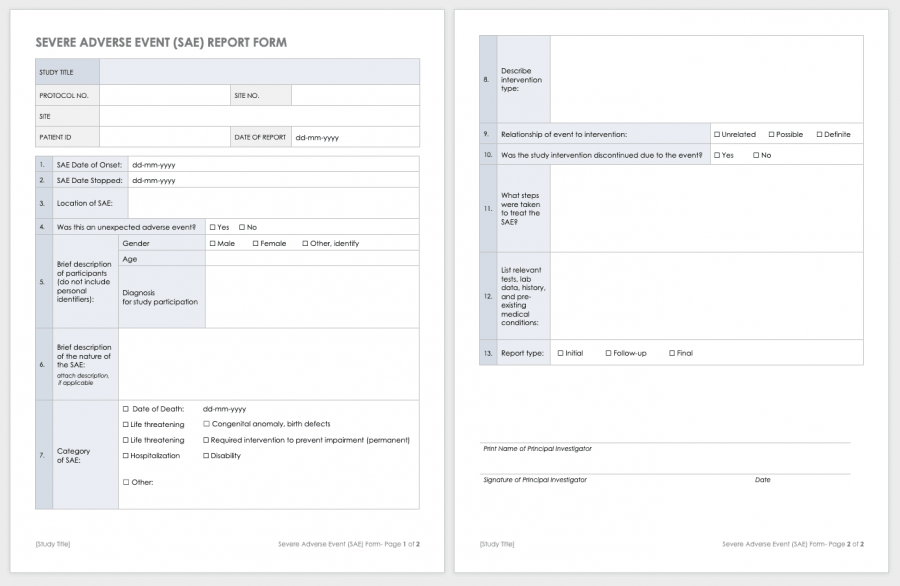
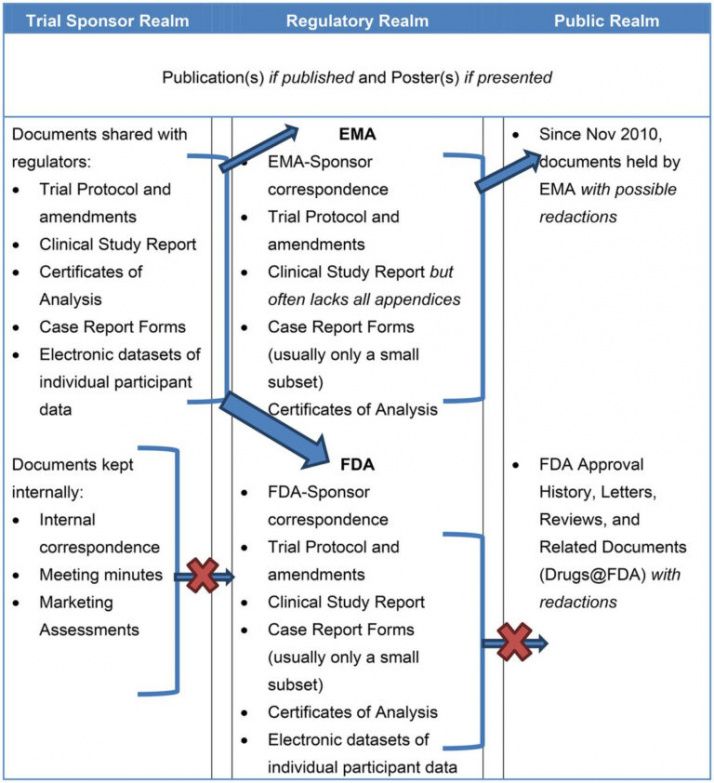
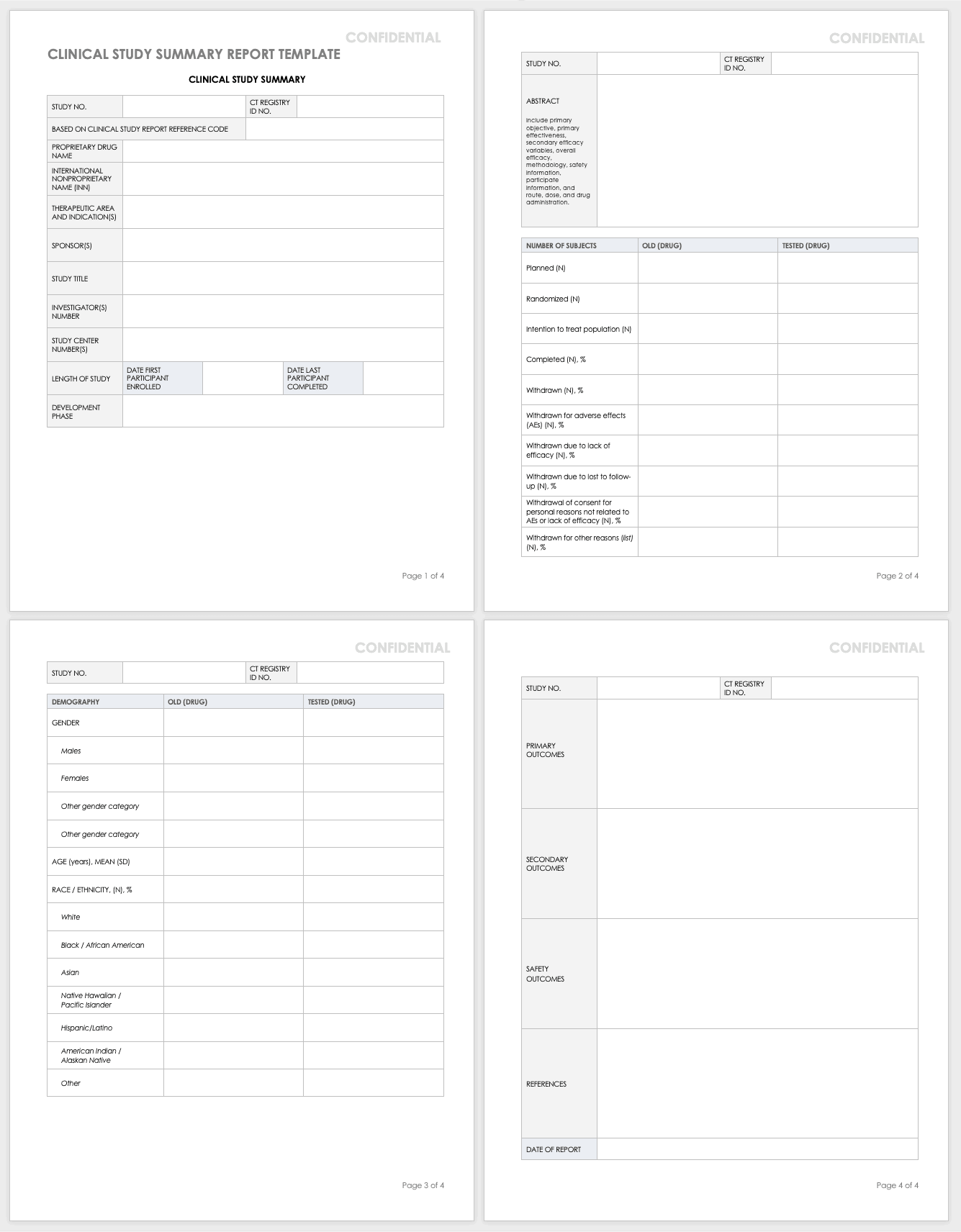
Clinical Study Report Template - Find protocol, data management, and monitoring templates for clinical trials funded by niams. Learn how to write a data and safety. It covers topics such as. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions.
This document provides recommendations for the structure and content of clinical study reports submitted to the fda. Learn how to write a data and safety. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. It covers topics such as. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as.
This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. Learn how to write a data and safety. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. It covers topics such as.
Clinical Study Report (CSR) Template Clinical Study Templates
Learn how to write a data and safety. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This.
(PDF) Clinical Study Report (CSR) Clinical Study Report (CSR)
This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. Learn how to write a data and safety. This document provides a note for guidance on the structure and content of clinical study reports.
Clinical Research Report Synopsis Templates At for Clinical Trial
It covers topics such as. Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This is an abbreviated clinical study.
Clinical Study Report PDF Cardiovascular Diseases Coronary Artery
Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. This document provides a note for guidance on the structure and.
Free Clinical Trial Templates Smartsheet
This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed.
Clinical Study Report Template PDF Sample Stableshvf
Learn how to write a data and safety. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. It covers topics such as. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. Find protocol, data management, and monitoring templates for clinical trials funded.
Structure AND Content OF Clinical Study Reports STRUCTURE AND CONTENT
This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. Find protocol, data management, and monitoring templates for clinical trials funded by niams. It covers topics such as. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the.
Free Clinical Trial Templates Smartsheet
Learn how to write a data and safety. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Find protocol, data management, and monitoring templates for clinical trials funded by niams. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine.
Clinical Study Report Cardiovascular Diseases Coronary Artery Disease
Learn how to write a data and safety. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. It covers topics such as. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document provides recommendations for the structure.
Clinical Trial Report Template TEMPLATES EXAMPLE TEMPLATES EXAMPLE
Learn how to write a data and safety. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This document provides a note for guidance on the structure and content.
This Document Provides A Harmonised Tripartite Guideline For The Structure And Content Of Clinical Study Reports For Registration Of.
This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Learn how to write a data and safety. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document provides recommendations for the structure and content of clinical study reports submitted to the fda.
Find Protocol, Data Management, And Monitoring Templates For Clinical Trials Funded By Niams.
It covers topics such as. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes.