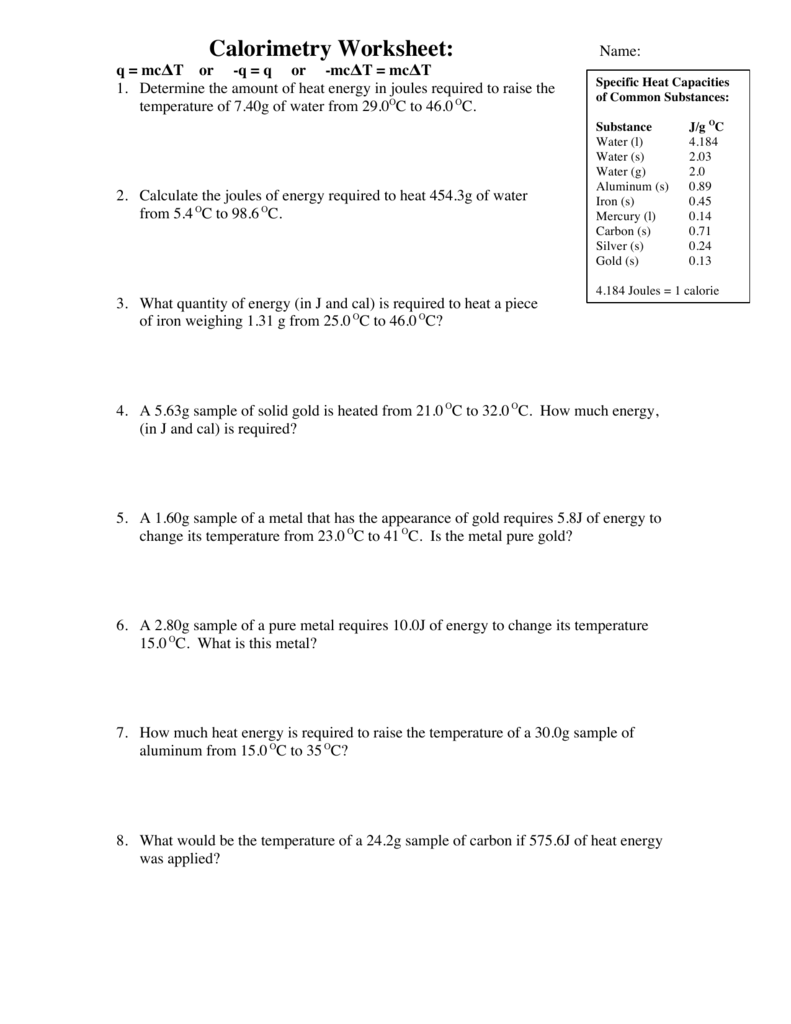
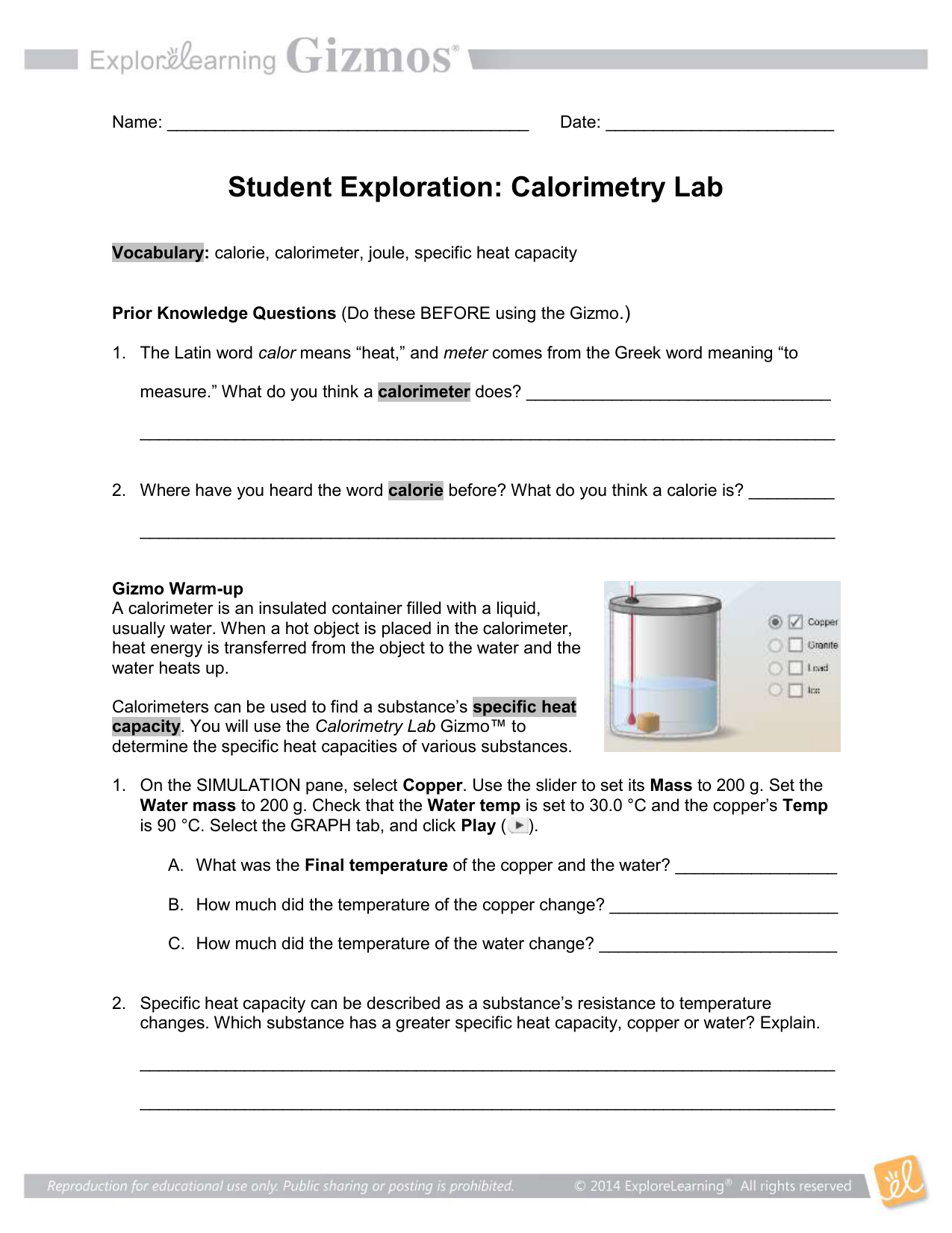
Calorimetry Worksheet Answers - The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. If the combustion of 0.285 moles of this compound causes the. Calorimetry questions and problems 1. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the.
Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. If the combustion of 0.285 moles of this compound causes the. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Calorimetry questions and problems 1. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water.
1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Calorimetry questions and problems 1. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. If the combustion of 0.285 moles of this compound causes the. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc?
Heat And Calorimetry Worksheets
Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry questions and problems 1. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. If the combustion of 0.285 moles.
Calorimetry Worksheet 1 Answers at tarscarletteblog Blog
Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry.
Calorimetry Worksheet Answer Key Foothill High School Worksheets
Calorimetry questions and problems 1. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in.
Calorimetry Practice Problems With Answers Calorimetry Pract
Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? If the combustion of 0.285 moles of this compound causes the. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams.
Calorimetry Worksheet Answer Key
If the combustion of 0.285 moles of this compound causes the. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of.
Calorimetry Problems Worksheet With Answers
The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. If the combustion of 0.285 moles of this compound causes the. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calorimetry questions and problems 1. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely.
Calorimetry Worksheet Answer Key
Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. Calorimetry questions and problems 1. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calorimetry.
Heat Capacity Calorimetry Worksheet answers NAME_________________PER
How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? If the combustion of 0.285 moles of this compound causes the. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a.
Calorimetry Questions And Answers
Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calculate the amount of heat transferred from the engine to the surroundings by one gallon.
Calorimetry Practice Problems With Answers Calorimetry Pract
Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 1) a compound is burned.
The Specific Heats Of Water And Iron Are 4.184 J/Goc And 0.44 J/Goc Respectively.
Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry questions and problems 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? If the combustion of 0.285 moles of this compound causes the.
1) A Compound Is Burned In A Bomb Calorimeter That Contains 3.00 L Of Water.
Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the.