Calculating Specific Heat Worksheet - Practice calculating specific heat capacity and energy using mc∆t formula. How many joules of heat are needed to raise the temperature of 150 kg of aluminum from 22°c to 55°c, if the specific heat of aluminum is 899. Use the formula q = (m)(δt)(cp) to solve the problems and show. A worksheet with 18 problems involving specific heat capacity and heat energy. M = 40 kg and δθ =. Calculate the energy transferred for each of these: Solve problems with iron, aluminum, wood, water, mercury, silver and. M = 0.5 kg and δθ = 20 °c (for copper) 2. M = 2 kg and δθ = 60 °c (for oil) 3.
M = 0.5 kg and δθ = 20 °c (for copper) 2. Use the formula q = (m)(δt)(cp) to solve the problems and show. Calculate the energy transferred for each of these: A worksheet with 18 problems involving specific heat capacity and heat energy. M = 2 kg and δθ = 60 °c (for oil) 3. Practice calculating specific heat capacity and energy using mc∆t formula. M = 40 kg and δθ =. How many joules of heat are needed to raise the temperature of 150 kg of aluminum from 22°c to 55°c, if the specific heat of aluminum is 899. Solve problems with iron, aluminum, wood, water, mercury, silver and.
Practice calculating specific heat capacity and energy using mc∆t formula. M = 0.5 kg and δθ = 20 °c (for copper) 2. M = 2 kg and δθ = 60 °c (for oil) 3. A worksheet with 18 problems involving specific heat capacity and heat energy. Use the formula q = (m)(δt)(cp) to solve the problems and show. How many joules of heat are needed to raise the temperature of 150 kg of aluminum from 22°c to 55°c, if the specific heat of aluminum is 899. M = 40 kg and δθ =. Calculate the energy transferred for each of these: Solve problems with iron, aluminum, wood, water, mercury, silver and.
How to Calculate Specific Heat 6 Steps (with Pictures) wikiHow
Solve problems with iron, aluminum, wood, water, mercury, silver and. Practice calculating specific heat capacity and energy using mc∆t formula. How many joules of heat are needed to raise the temperature of 150 kg of aluminum from 22°c to 55°c, if the specific heat of aluminum is 899. M = 40 kg and δθ =. M = 2 kg and.
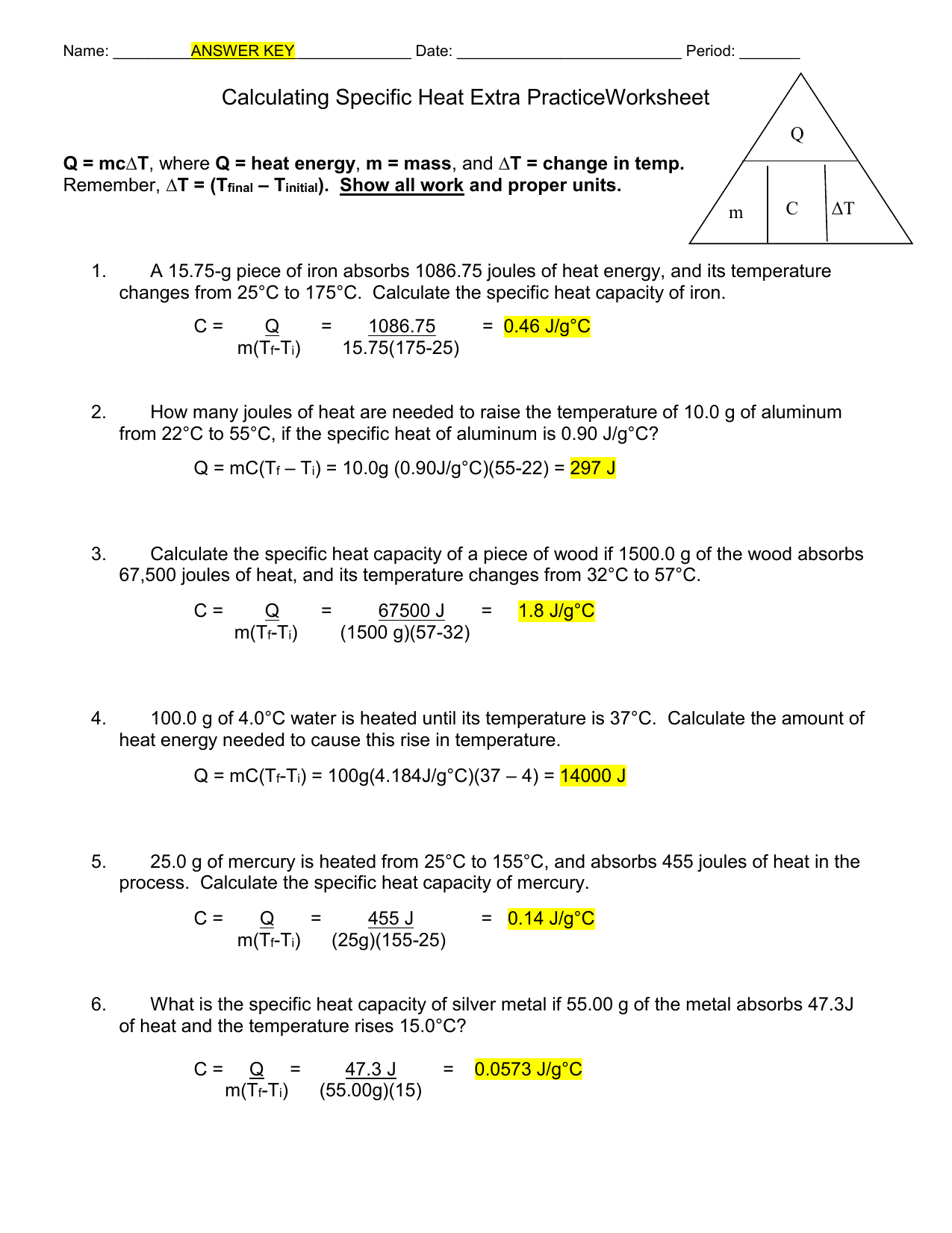
Calculating Specific Heat Extra Practice Worksheet Specific
M = 0.5 kg and δθ = 20 °c (for copper) 2. Practice calculating specific heat capacity and energy using mc∆t formula. Solve problems with iron, aluminum, wood, water, mercury, silver and. How many joules of heat are needed to raise the temperature of 150 kg of aluminum from 22°c to 55°c, if the specific heat of aluminum is 899..
Calculating Heat And Specific Heat Worksheet
M = 40 kg and δθ =. Solve problems with iron, aluminum, wood, water, mercury, silver and. M = 0.5 kg and δθ = 20 °c (for copper) 2. Practice calculating specific heat capacity and energy using mc∆t formula. How many joules of heat are needed to raise the temperature of 150 kg of aluminum from 22°c to 55°c, if.
Calculating Specific Heat Worksheet Pro Worksheet
Solve problems with iron, aluminum, wood, water, mercury, silver and. M = 2 kg and δθ = 60 °c (for oil) 3. Use the formula q = (m)(δt)(cp) to solve the problems and show. Practice calculating specific heat capacity and energy using mc∆t formula. M = 0.5 kg and δθ = 20 °c (for copper) 2.
Calculating Heat And Specific Heat Worksheets
Solve problems with iron, aluminum, wood, water, mercury, silver and. Calculate the energy transferred for each of these: Practice calculating specific heat capacity and energy using mc∆t formula. M = 0.5 kg and δθ = 20 °c (for copper) 2. M = 40 kg and δθ =.
Calculating Specific Heat Worksheet Printable And Enjoyable Learning
A worksheet with 18 problems involving specific heat capacity and heat energy. M = 0.5 kg and δθ = 20 °c (for copper) 2. How many joules of heat are needed to raise the temperature of 150 kg of aluminum from 22°c to 55°c, if the specific heat of aluminum is 899. Calculate the energy transferred for each of these:.
30++ Calculating Specific Heat Worksheet Worksheets Decoomo
M = 40 kg and δθ =. Practice calculating specific heat capacity and energy using mc∆t formula. Calculate the energy transferred for each of these: How many joules of heat are needed to raise the temperature of 150 kg of aluminum from 22°c to 55°c, if the specific heat of aluminum is 899. A worksheet with 18 problems involving specific.
Calculating Heat And Specific Heat Worksheet
How many joules of heat are needed to raise the temperature of 150 kg of aluminum from 22°c to 55°c, if the specific heat of aluminum is 899. M = 0.5 kg and δθ = 20 °c (for copper) 2. M = 2 kg and δθ = 60 °c (for oil) 3. Practice calculating specific heat capacity and energy using.
Specific Heat Worksheets WorksheetsGO
How many joules of heat are needed to raise the temperature of 150 kg of aluminum from 22°c to 55°c, if the specific heat of aluminum is 899. Solve problems with iron, aluminum, wood, water, mercury, silver and. M = 2 kg and δθ = 60 °c (for oil) 3. Practice calculating specific heat capacity and energy using mc∆t formula..
Specific Heat Calculations KEY Calculating Specific Heat
Solve problems with iron, aluminum, wood, water, mercury, silver and. M = 40 kg and δθ =. M = 0.5 kg and δθ = 20 °c (for copper) 2. Practice calculating specific heat capacity and energy using mc∆t formula. M = 2 kg and δθ = 60 °c (for oil) 3.
Solve Problems With Iron, Aluminum, Wood, Water, Mercury, Silver And.
M = 40 kg and δθ =. Use the formula q = (m)(δt)(cp) to solve the problems and show. A worksheet with 18 problems involving specific heat capacity and heat energy. M = 2 kg and δθ = 60 °c (for oil) 3.
How Many Joules Of Heat Are Needed To Raise The Temperature Of 150 Kg Of Aluminum From 22°C To 55°C, If The Specific Heat Of Aluminum Is 899.
Calculate the energy transferred for each of these: Practice calculating specific heat capacity and energy using mc∆t formula. M = 0.5 kg and δθ = 20 °c (for copper) 2.