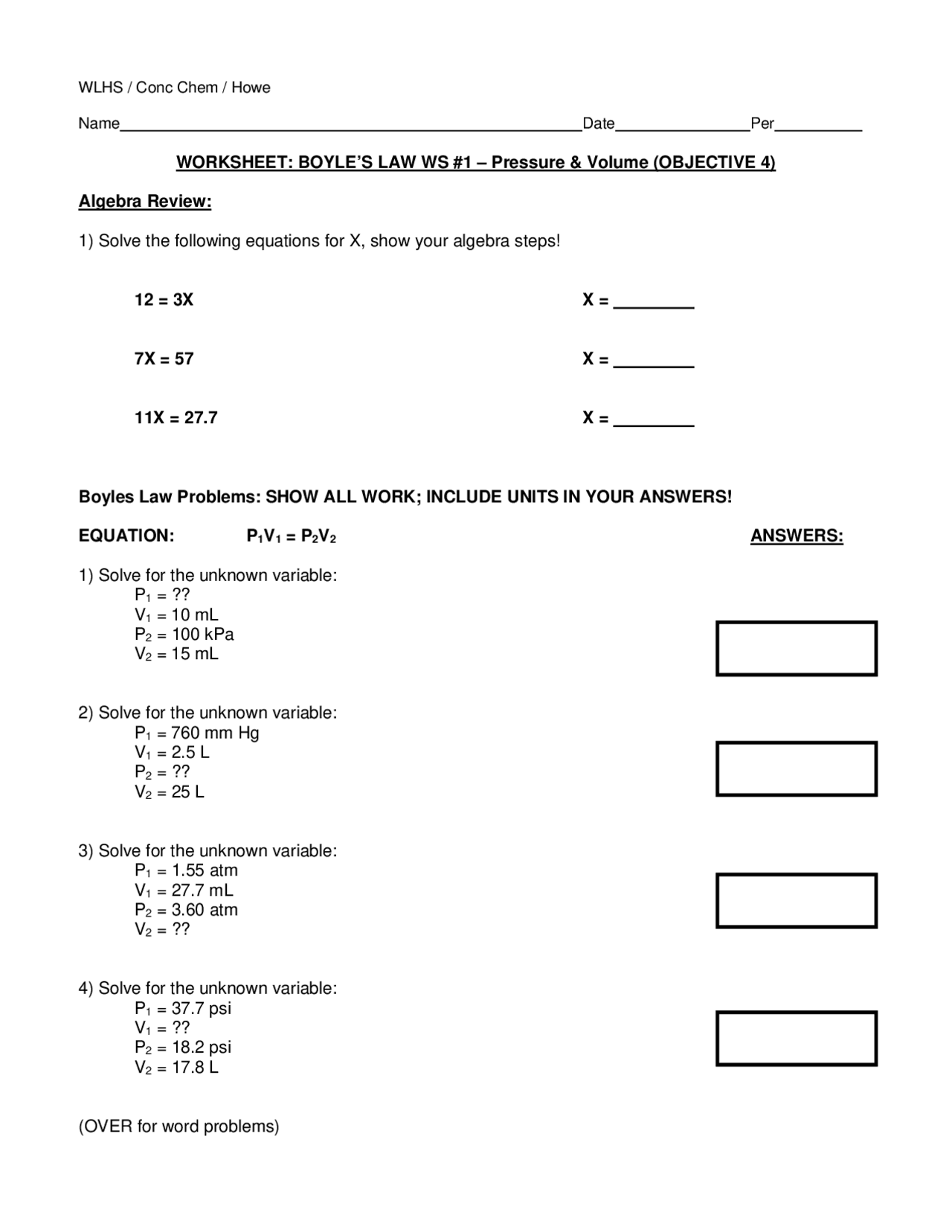
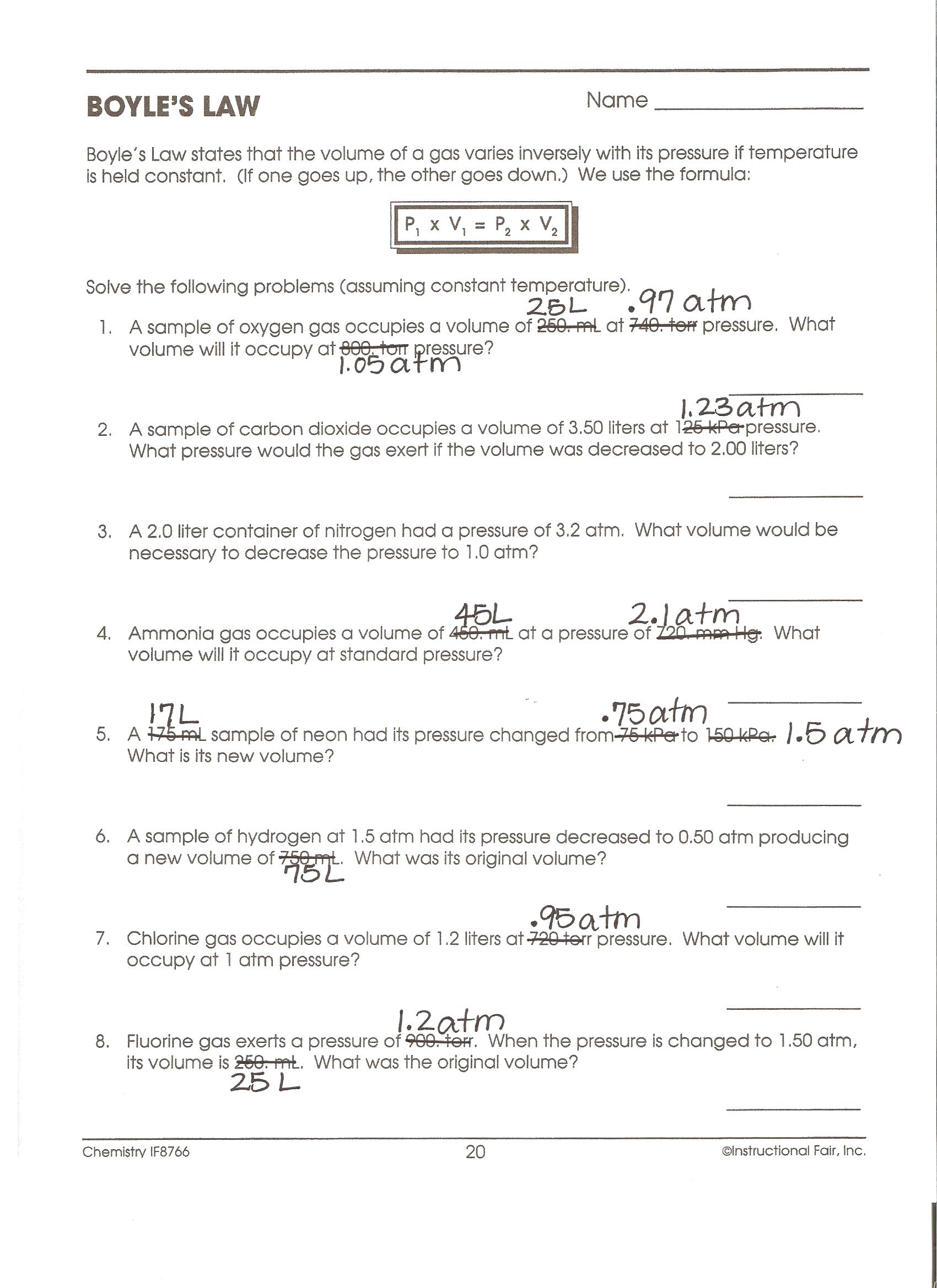
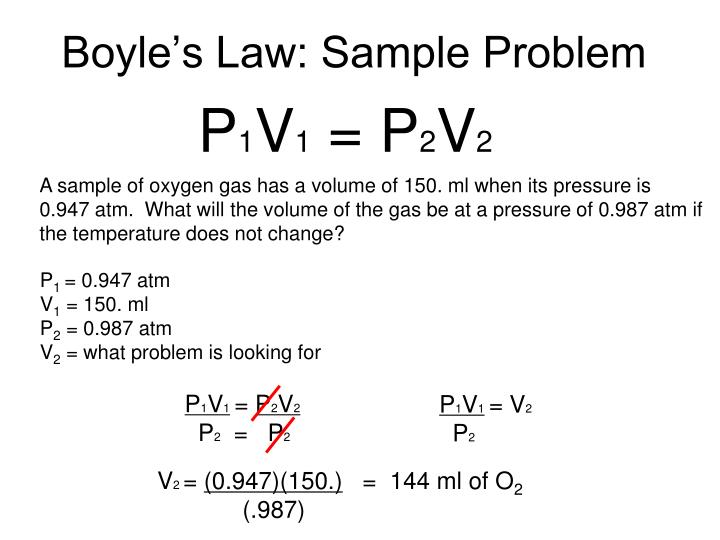
Boyle S Law Worksheet Answers - Boyles' law to answer the following questions: P 1 v 1 = p 2 v 2 1. Boyle’s law practice problems boyle’s law states: If a gas at 75.0 °c occupies 13.60 liters at a pressure of 1.00 atm,. Boyles’ law worksheet use boyles’ law to answer the following questions: 1) 1.00 l of a gas at standard temperature and pressure is. These two variables are inversely proportional. Robert boyle observed the relationship between the pressure and volume for a gas sample. 1) 1.00 l of a gag at standard temperature and pressur is compressed to 6473 rnlo what is the. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional.
1) solve the following equations for x, show your algebra. Boyles’ law worksheet use boyles’ law to answer the following questions: These two variables are inversely proportional. Boyle’s law practice problems boyle’s law states: Boyles' law to answer the following questions: When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. 1) 1.00 l of a gag at standard temperature and pressur is compressed to 6473 rnlo what is the. If a gas at 75.0 °c occupies 13.60 liters at a pressure of 1.00 atm,. 1) 1.00 l of a gas at standard temperature and pressure is. P 1 v 1 = p 2 v 2 1.
These two variables are inversely proportional. If a gas at 75.0 °c occupies 13.60 liters at a pressure of 1.00 atm,. 1) 1.00 l of a gag at standard temperature and pressur is compressed to 6473 rnlo what is the. P 1 v 1 = p 2 v 2 1. Boyle’s law practice problems boyle’s law states: Robert boyle observed the relationship between the pressure and volume for a gas sample. 1) 1.00 l of a gas at standard temperature and pressure is. 1) solve the following equations for x, show your algebra. Boyles’ law worksheet use boyles’ law to answer the following questions: Boyles' law to answer the following questions:
WORKSHEET BOYLE'S LAW WS 1 Pressure & Volume Summaries Law
1) solve the following equations for x, show your algebra. These two variables are inversely proportional. P 1 v 1 = p 2 v 2 1. If a gas at 75.0 °c occupies 13.60 liters at a pressure of 1.00 atm,. 1) 1.00 l of a gas at standard temperature and pressure is.
Boyle's Law Practice Problems Worksheet Answers
1) 1.00 l of a gas at standard temperature and pressure is. 1) solve the following equations for x, show your algebra. If a gas at 75.0 °c occupies 13.60 liters at a pressure of 1.00 atm,. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. Boyles’ law worksheet use boyles’ law.
Boyle’s Law Problems Answers Boyle’s Law Problems ANSWERS Boyle’s Law
1) 1.00 l of a gag at standard temperature and pressur is compressed to 6473 rnlo what is the. Robert boyle observed the relationship between the pressure and volume for a gas sample. Boyles' law to answer the following questions: Boyle’s law practice problems boyle’s law states: 1) 1.00 l of a gas at standard temperature and pressure is.
Boyle's Law Questions And Answers Boyle S Law Worksheet Answ
Boyles' law to answer the following questions: If a gas at 75.0 °c occupies 13.60 liters at a pressure of 1.00 atm,. Boyle’s law practice problems boyle’s law states: Boyles’ law worksheet use boyles’ law to answer the following questions: These two variables are inversely proportional.
Boyle's Law Questions And Answers
Robert boyle observed the relationship between the pressure and volume for a gas sample. Boyle’s law practice problems boyle’s law states: Boyles' law to answer the following questions: 1) solve the following equations for x, show your algebra. These two variables are inversely proportional.
20++ Boyle's Law Practice Problems Worksheet Answers Worksheets Decoomo
Boyles’ law worksheet use boyles’ law to answer the following questions: If a gas at 75.0 °c occupies 13.60 liters at a pressure of 1.00 atm,. Boyle’s law practice problems boyle’s law states: 1) 1.00 l of a gas at standard temperature and pressure is. When ____________ temperature is held constant, the pressure and volume of a gas are ____________.
Boyle's Law And Charles Law Worksheets Answers
Boyles' law to answer the following questions: These two variables are inversely proportional. 1) solve the following equations for x, show your algebra. Boyle’s law practice problems boyle’s law states: 1) 1.00 l of a gas at standard temperature and pressure is.
Boyle's Law Examples Problems
Boyle’s law practice problems boyle’s law states: Boyles' law to answer the following questions: P 1 v 1 = p 2 v 2 1. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. 1) 1.00 l of a gas at standard temperature and pressure is.
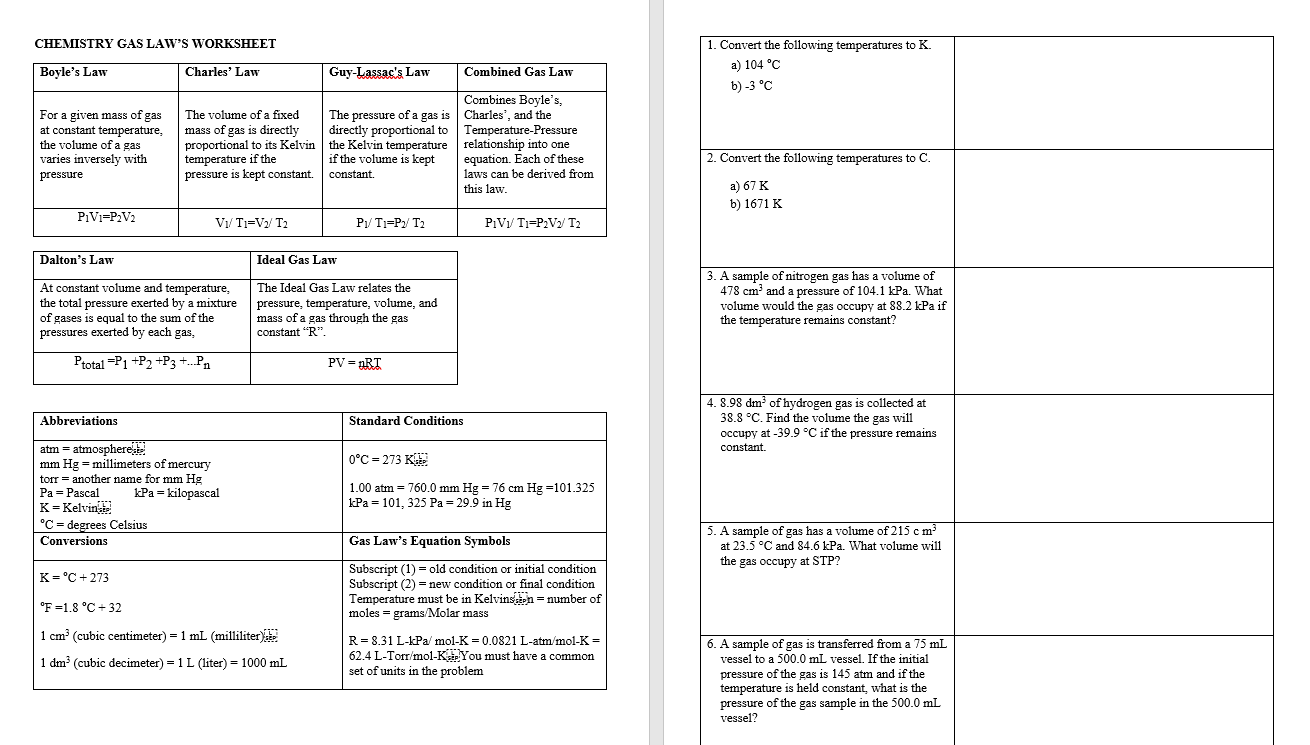
Solved CHEMISTRY GAS LAW'S WORKSHEET Boyle's Law Charles'
Boyles' law to answer the following questions: 1) solve the following equations for x, show your algebra. Boyle’s law practice problems boyle’s law states: If a gas at 75.0 °c occupies 13.60 liters at a pressure of 1.00 atm,. These two variables are inversely proportional.
Boyle's Law Worksheets With Answers Printable Word Searches
Boyle’s law practice problems boyle’s law states: These two variables are inversely proportional. P 1 v 1 = p 2 v 2 1. Boyles' law to answer the following questions: When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional.
1) Solve The Following Equations For X, Show Your Algebra.
Boyles' law to answer the following questions: Boyles’ law worksheet use boyles’ law to answer the following questions: These two variables are inversely proportional. P 1 v 1 = p 2 v 2 1.
Boyle’s Law Practice Problems Boyle’s Law States:
When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. If a gas at 75.0 °c occupies 13.60 liters at a pressure of 1.00 atm,. 1) 1.00 l of a gas at standard temperature and pressure is. 1) 1.00 l of a gag at standard temperature and pressur is compressed to 6473 rnlo what is the.